Compare and Explain the Different Properties of Graphite and Diamond
The presence of layers means that atoms can slide over each other easily. Since Diamonds and Graphite have quite different properties they are used for very.
Difference Between Diamond And Graphite Definition Properties Uses
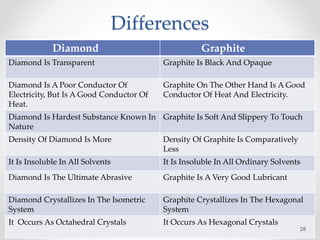
Some differences that Diamonds and Graphite have are that Diamonds are very hard whereas Graphite is very soft and easy to break.
. Furthermore diamond is the hardest naturally occurring material on earth but graphite and fullerene have comparatively low hardness. The planar structure of graphite allows electrons to move easily within the planes. Graphite has planar hexagonal layers of carbon atoms held together by weak Vander Waals forces and C is.
Another important physical difference is their hardness. Each carbon atom bonds to 4 other carbon atoms WHILST Graphite. Properties - 1 Methane is a Gas while diamond is a solid at room temperature.
Difference between the electrical conductivity of diamond and graphite are discussed as follows. Graphite is very soft and has a hardness of 1 to 2 on this scale. The hardness of minerals is compared using the Mohs Hardness Scale a relative scale numbered 1 softest to 10 hardest.
Yet diamond is the hardest mineral known to man 10 on the Mohs scale and graphite is one of the softest less than 1 on the Mohs scale. By Rudina El Dokani Science 9 1 Mrs. Graphite also has a lower density 2266 grams per cubic centimeter than diamond.
The presence of layers means that atoms can slide over each other easily. However the graphites particles join to the three atoms of carbon and get associated with the plates that are parallel to each other. The key difference between diamond graphite and fullerene is that diamond has a diamond cubic crystal structure and graphite has a hexagonal crystal structure while fullerene occurs as a large spheroidal molecule.
2 It is made up of tetrahedral units. They are chemically identical but very different physically which is why they are called polymorphous. Diamond does not conduct electricity because it has no charged particles that are free to move.
Diamond atoms have a rigid 3 dimensional structure with each atom carefully loaded with each other as well as connected to 4 other carbon atoms. Thus diamond bears more of a tetrahedral structure whereas graphite takes the form of layers. This makes diamond extremely hard.
3 In diamond each carbon atom is s p 3 hybridized and is bonded to four other carbon atoms through a sigma bond. Each carbon atom bonds to 3 other carbon atoms. Diamond is extremely hard because it is a giant covalent structure with many strong covalent bonds.
Graphene on the other hand is the strongest material ever recorded more than three hundred times stronger than A36 structural steel at 130 gigapascals and more than forty times stronger than diamond. Graphite is opaque and metallic- to earthy-looking while diamonds are transparent and brilliant. The diamond moleculesCrystal System is Isometric are shaped like perf View the full answer.
In diamond strong three-dimensional networks are formed due to the. 1 It has a layered structure. In diamond C atom is s p 3 hybridised due to tetrahedral structure.
5 rows Diamond. In graphite each carbon atom is covalently bonded to only three neighboring carbon atoms and these form layers of hexagonal network which are separated by a large distance. Diamond and also graphite are chemically the same both made up of the element carbon however they have entirely different atomic and also crystal frameworks.
DIAMOND GRAPHITE 1 It has a crystalline structure. Due to graphites planar structure its thermal acoustic and electronic properties are highly anisotropic meaning that phonons travel. The geometry is planar C-C bond length is 154 pm.
The difference in the properties of diamond and graphite can be easily explained in terms their structures. It has a 3 -Dimensional structure in which all the Carbon atoms are sp3 hybridized and each Carbon atom is bonded to 4 other Carbon atoms. What are 3 differences between diamond and graphite.
Graphite is also made of only carbon atoms and is also a giant structure but it is formed of layers where each carbon atom has a strong covalent bond to 3 other carbons. 2 It is made up of tetrahedral units. Fourth electron forms pi bond.
Diamond and graphite are two allotropic forms of carbon and their crystal structure is different from each other as shown in figure. Graphite It has crystalline nature. Diamond has four covalent bonds around one carbon atom.
The particles of Diamond enter the four atoms of carbon in a gem frame. 2 It has a planar geometry. But Diamond does not exist as a discrete molecule.
In diamond there is a three dimensional network of strong covalent bonds. Nicholl Tuesday December 17th Compare and contrast the properties of diamond and graphite Why do Diamonds and Graphite have such different properties. Graphite does conduct electricity because it has delocalised electrons.
Posted by Sharif Khan on 1st Feb 2021. Graphite has a planar structure. Diamonds are the hardest known natural.
The fourth valence electrons. As a result diamond is the ultimate abrasive whereas graphite is an excellent lubricant. Each carbon atom bonds to 3 other carbon atoms.
Diamond is vary hard whereas graphite is soft. For example Graphite and diamond are two different allotropes of carbonExplain the difference in properties of diamond and graphite on the basis of their structures. Thus graphites use as a lubricant.
They are both pure carbon. Graphite has three covalent bonds around one carbon atom. It has layered structure Each C is s p 3 hybridzed and forms 4 covalent bonds with neighboring C atoms.
Precious stone and graphite have shift structures which represent their diverse properties and both are pure carbon. Because it is hard diamond is used in high speed cutting tools eg diamond-tipped saws. Diamond has a face-centered cubic crystal structure.
Also a Diamonds density is greater than that of Graphite. 2 Methane is highly flammable while diamond is not. Because of hardness diamond is used in making cutting and grinding tools.
The primary structural difference between graphite diamond is that the way the carbon atoms are arranged to form the physical crystal structures. The geometry is tetrahedral. Thus diamond bears more of a tetrahedral structure whereas graphite takes the form of layers.
Difference between the electrical conductivity of diamond and graphite. Both diamond and graphite have a very simple chemical composition. Each C atom is s p 2 hybridized and forms 3 sigma bonds with 3 other C atoms.
Each carbon atom bonds to 4 other carbon atoms WHILST Graphite. 1 It has a layered structure. 1 It has a crystalline structure.

Explain The Difference In Properties Of Diamond And Graphite On The Basis Of Their Structures Target Batch

Explain The Difference In Properties Of Diamond And Graphite On The Basis Of Their Structures
Comments
Post a Comment